Use Case
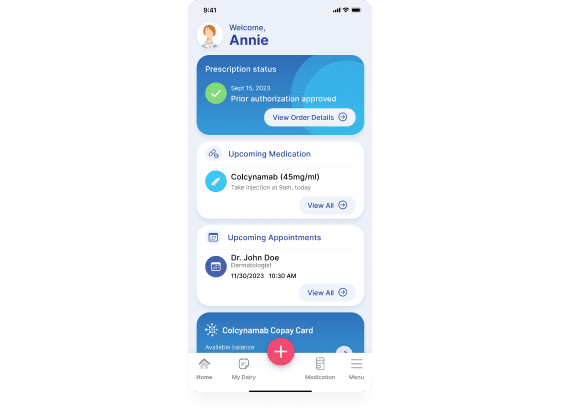
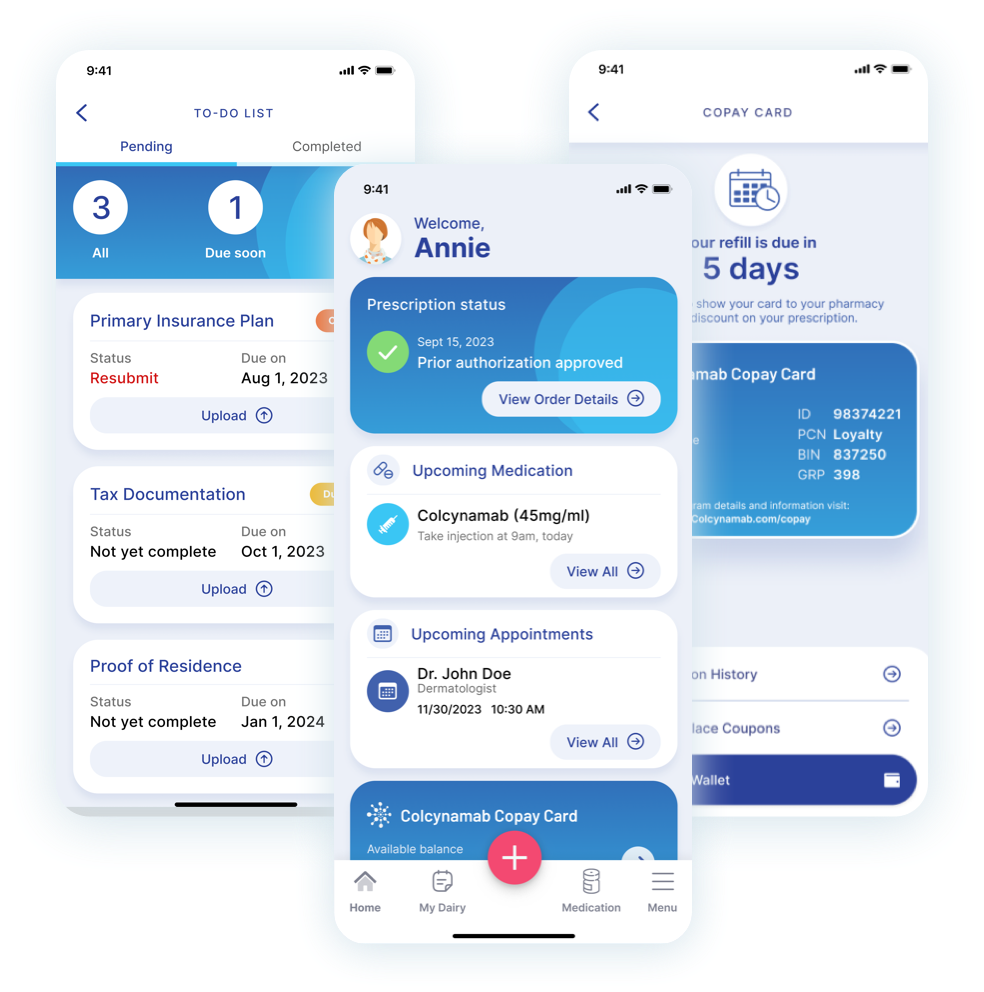
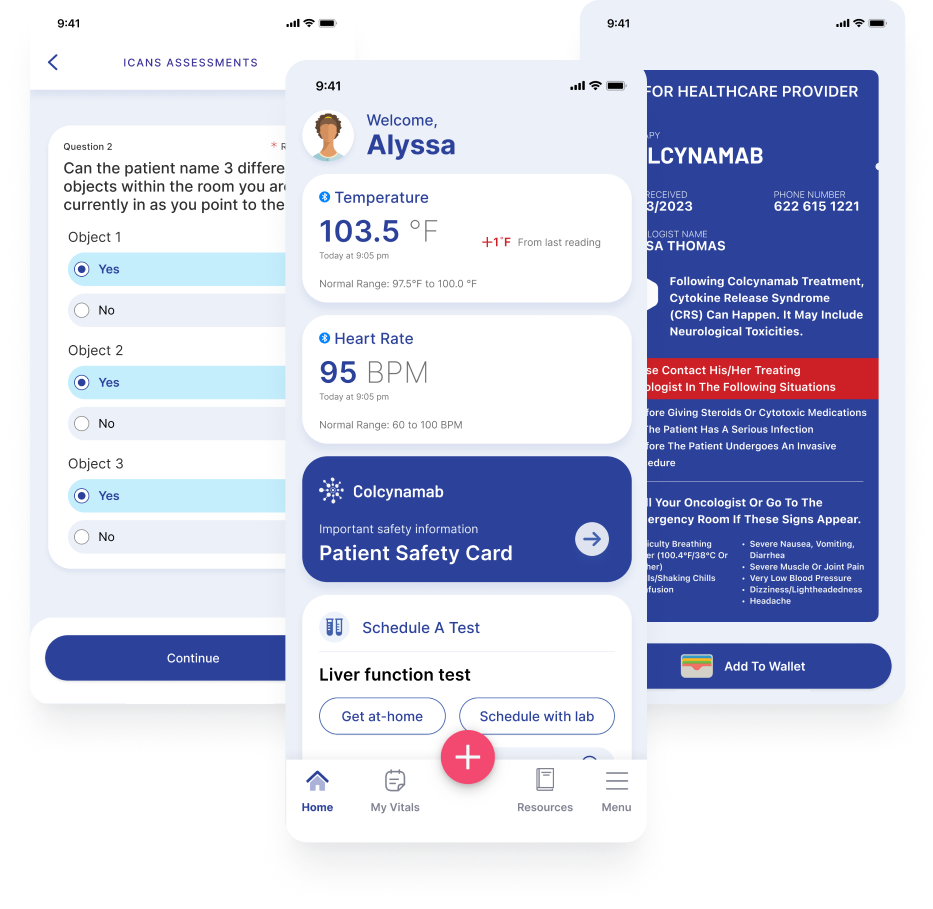
Support patients using at-home subcutaneous injections
See how our Disease Management Solution can be easily configured with a SubQ module to ease the burden of therapy for patients.
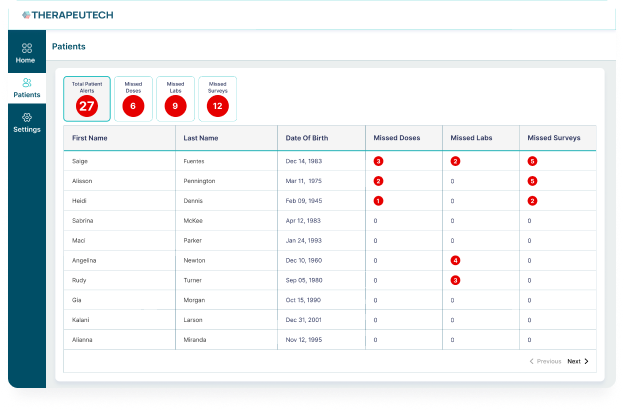
- Patient support services
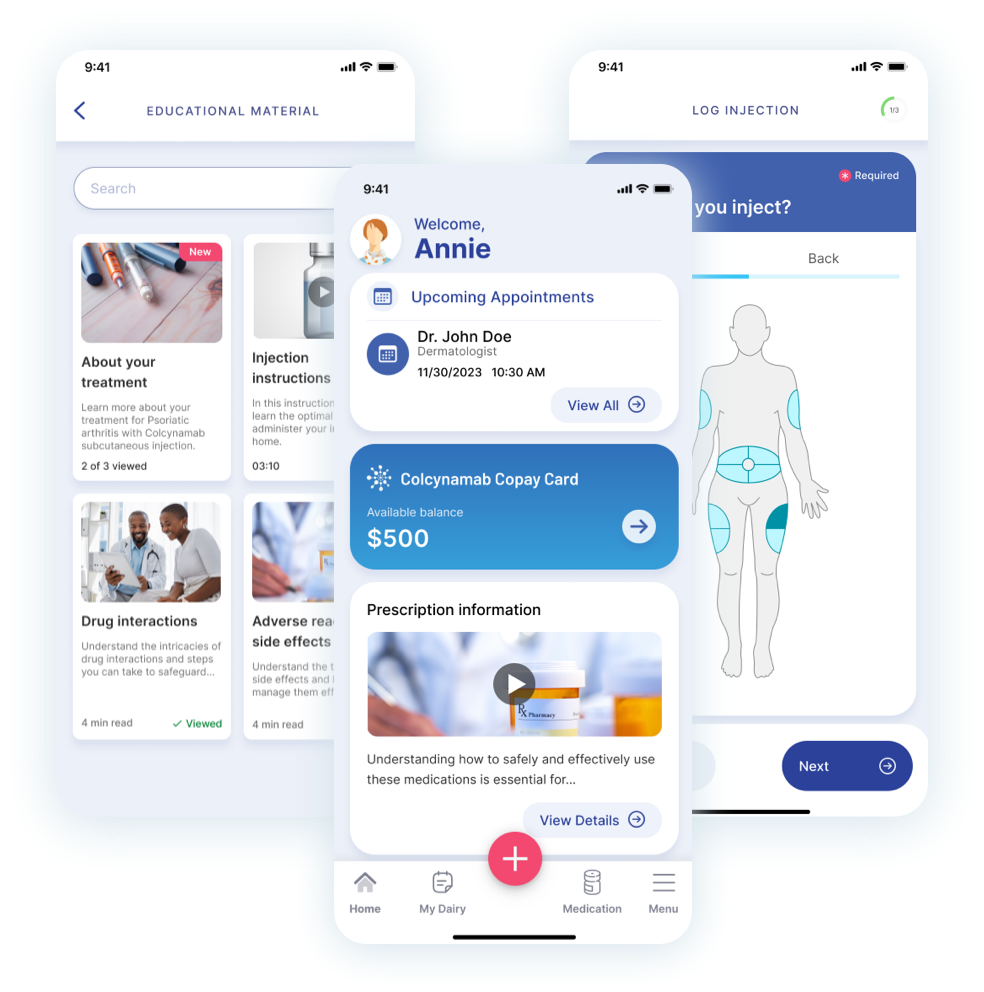
- Step-by-step administration guide
- Site tracking and rotation
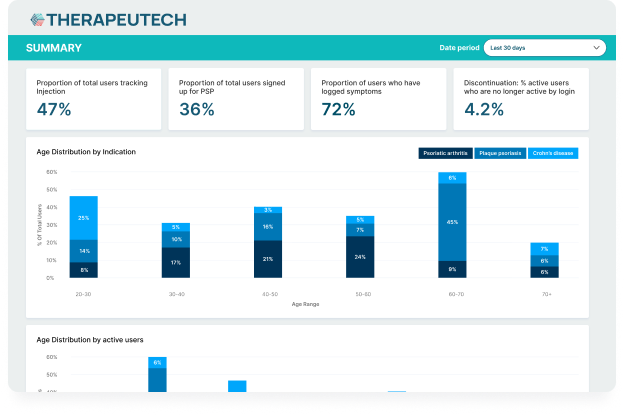
- Real-world data and feedback