New Data: Sanofi and BrightInsight drive significant gains in adherence and persistence
View the Results
BrightInsight
The trusted partner and de facto platform driving value in digital health

We’re proud to be the trusted leader in digital health, delivering measurable results for flagship brands in the largest markets.









Top life sciences companies trust the BrightInsight Platform with their most important digital offerings, including companion apps, algorithms, connected combination products, healthcare provider interfaces and other Software as a Medical Device (SaMD) across therapy areas.

14
SaMD developed
11
therapy areas
6/10
top biopharma companies
have partnered with us
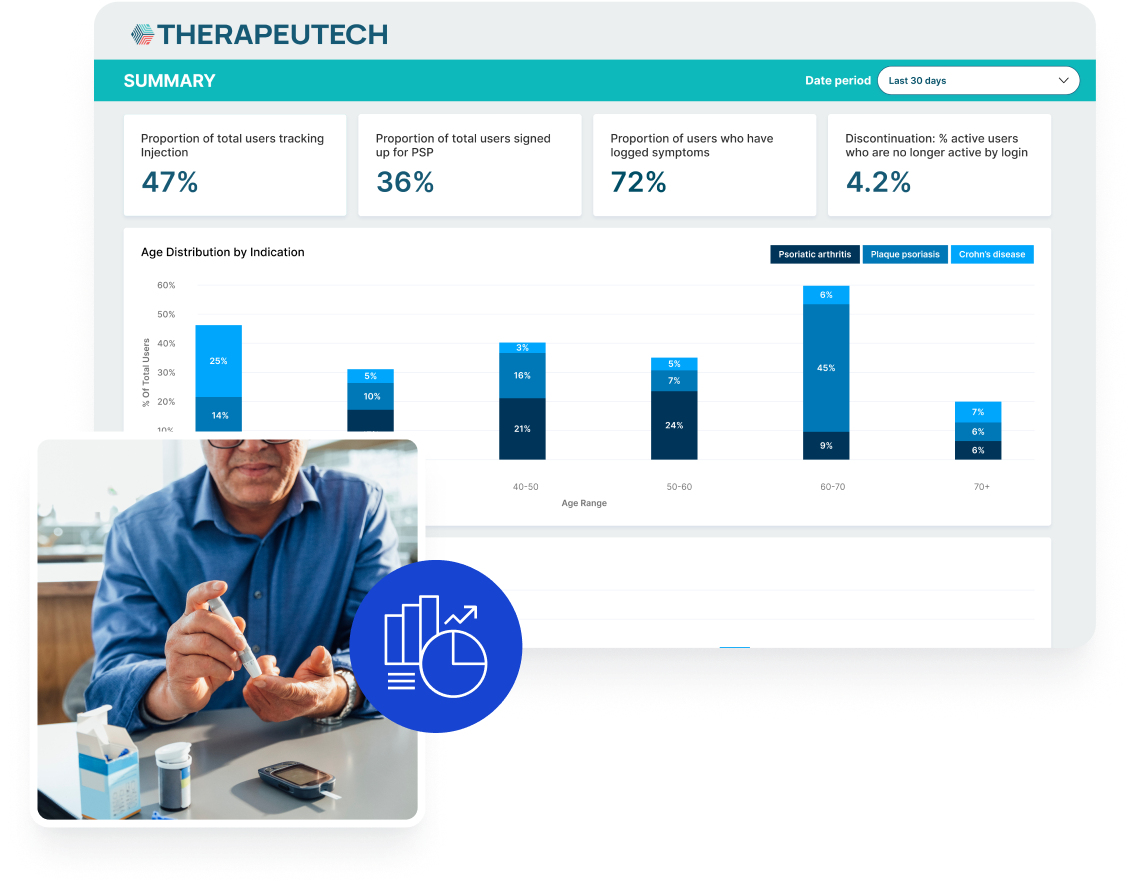

Read how the Hizentra app, built on the BrightInsight® Platform, gained high user adoption and an over 70% retention rate from 90 days to 12 months.
Read Now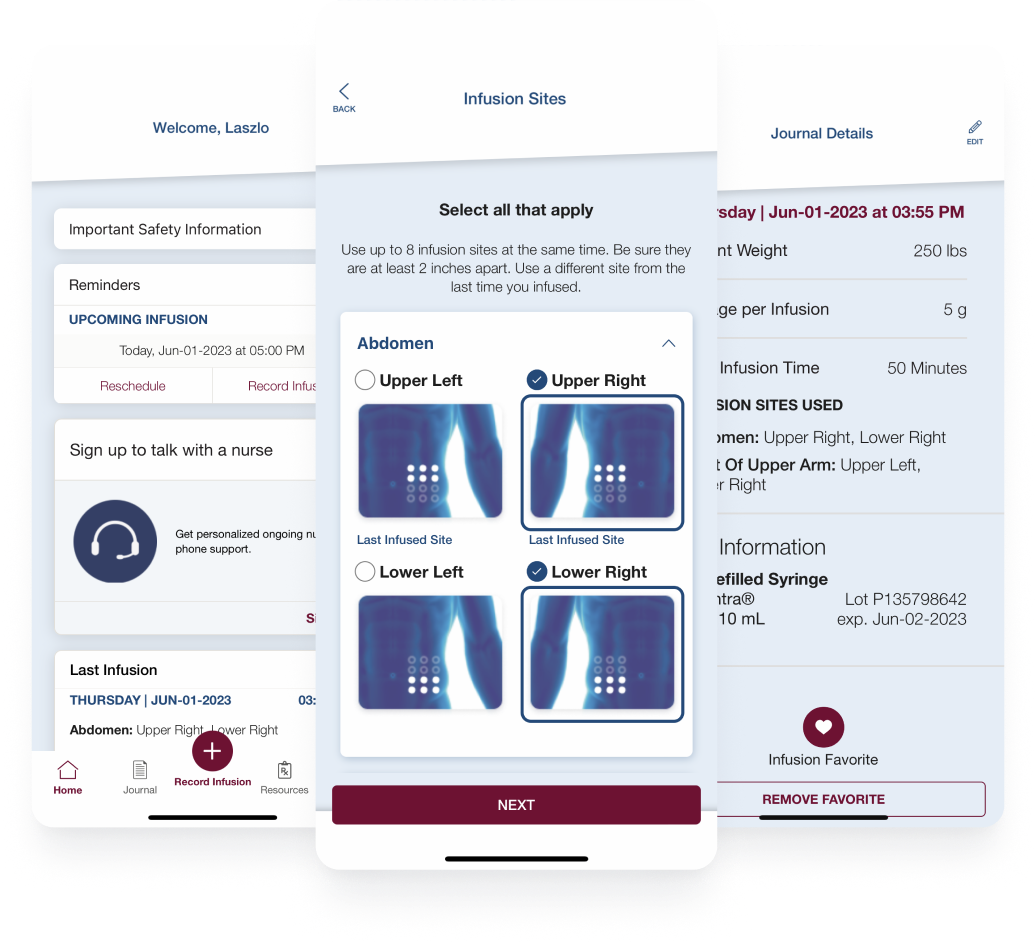








Browse our latest white papers, case studies, webinars and more

Contact us today to start a conversation about how BrightInsight can help on your digital health journey.
Contact us