Case Study
New capabilities, new regions, unparalleled app engagement
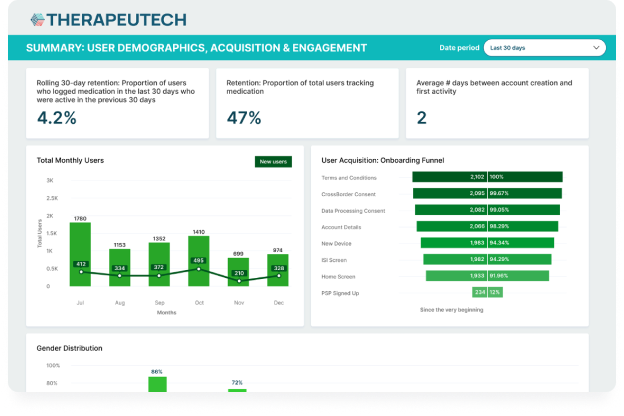
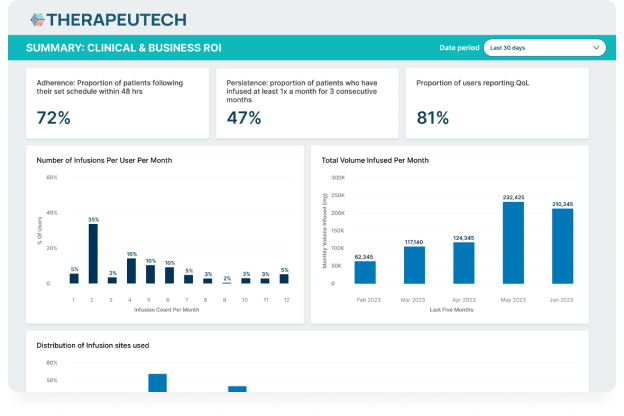
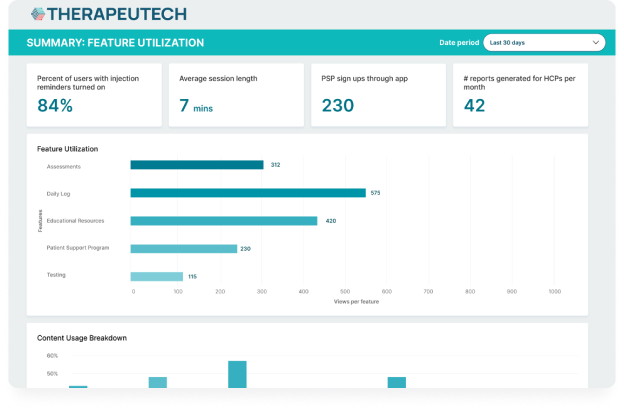
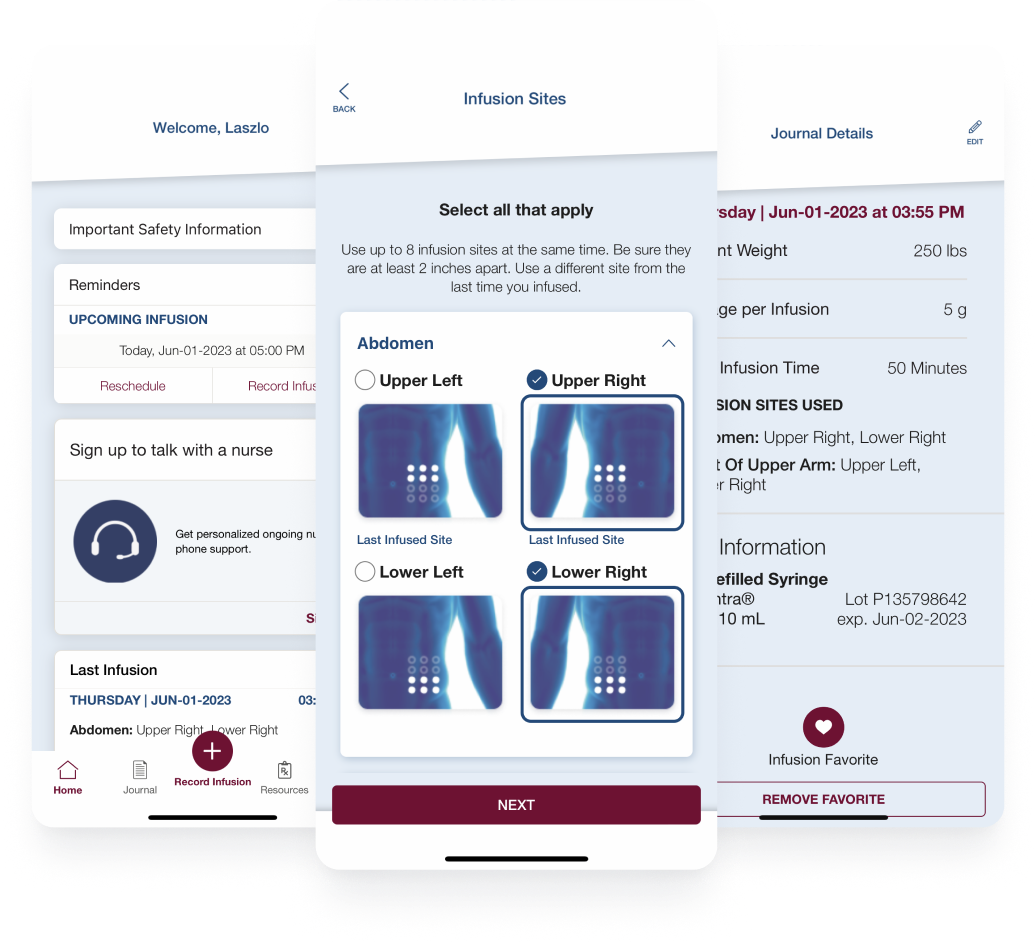
CSL Behring’s Hizentra app, built on the BrightInsight Platform, demonstrates our ability to efficiently help our biopharma partners launch and scale their drug companion apps.
- New functionality like a nurse educator program and more flexible patient flows
- Expansion into Latin America and Canada, in addition to the US and Japan
- App adoption rate of over 25% of the brand’s US patient population