Chronic conditions are becoming more prevalent, along with the rapidly aging global population. At the same time, the continuous evolution towards value-based care drives precision medicine (PM)—delivering the right treatment to the right patient at the right time—as the foremost healthcare strategy going forward. Likewise, biopharmaceutical (biopharma) and medical technology (medtech) companies increasingly implement patient-specific development approaches, embracing more tailored therapeutic interventions.
The biopharma and medtech industries are in a state of flux as companies manage the agile innovation and regulatory compliance pendulum to stand out in an increasingly competitive value-driven landscape. Furthermore, as healthcare becomes democratized, patients are emerging as key decision-makers. With the entry of non-traditional tech companies, such as Apple, Amazon, and Google, digitally-enabled patients drive biopharma and medtech to go beyond traditional products and boundaries.
Amid the fragmentation/disruption, pioneering companies offer cutting-edge digital technologies, e.g., artificial intelligence, Big Data and analytics, cloud, mobile, blockchain, and the Internet of Things (IoT), to build digital convergence across the global clinical trial landscape and innovation ecosystem. Emerging technology integrations address various bottlenecks of the value chain through novel platforms, functional point solutions, and evolving business models. However, current solutions mostly involve proprietary software providing siloed information with limited content, rendering suboptimal information for an evidence-based decision-making framework, i.e., they are not conducive to PM.
The innovation spotlight is on game-changing, tech-integrated platform solutions that alter the status-quo by highlighting novel monetization models for digital health products and innovation.1
Frost & Sullivan estimates the global precision medicine informatics service market crossing the $5 billion mark by the end of 2020.2
Headquartered in Silicon Valley, BrightInsight provides the leading regulated Internet of Things (IoT) platform, the BrightInsight Platform, to accelerate regulated digital health innovation for the global biopharma and medtech industries.
The company has team members around the globe, including in the United States’ (US) East Coast—New Jersey—and Europe (EU)—Hägglingen, Switzerland, and Copenhagen, Denmark. It is currently building a delivery center in London, England, the United Kingdom, and expanding into Asia.
BrightInsight conforms to global security, privacy, and regulatory standards such as a Quality Management System that is ISO 13485:2016 certified HITRUST CSF® v9.1, GDPR, and HIPAA. Beyond the US and EU, the regulated IoT solution is available and cleared, from a privacy perspective, in more than 30 countries, with regulatory approval soon to follow across global markets.
Current customers include leading multinationals like Novo Nordisk, Roche and CSL Behring.
BrightInsight: Pioneering Leadership
The BrightInsight Platform launched in 2018 as a part of the leading multinational manufacturing services provider, Flex. The business spun as a standalone, venture-backed company in the summer of 2019.
Kal Patel, MD, BrightInsight’s CEO and Co-founder, made two key observations when arriving at Flex. First, a massive shift towards IoT in biopharma and medtech as these industries realize traditional research and development investments show diminishing returns. IoT is the way for them to stay competitive and relevant. Second, innovators do not understand the massive challenge of developing a homegrown IoT infrastructure. Dr. Patel experienced this hardship first-hand when leading digital health at Amgen.
Prior to BrightInsight, biopharma and medtech companies looking to launch digital health solutions had no choice but to try and build their custom platform from the ground up, with all of the regulatory, security, and infrastructure requirements to meet each market’s regulations. This effort can take a couple of years and costs from $10 to $20 million, and that is only for one product in one region.
This problem is solved through a pre-built, robust platform that meets the rigor that biopharma and medtech companies require across security, privacy, quality and regulatory requirements.
BrightInsight leveraged its management team’s subject matter expertise and technological know-how to deliver a unified digital solution for regulated digital health innovation.
Mission Innovation: 2020 and Beyond
BrightInsight envisions bringing the power of digital technology to healthcare to transform patient outcomes globally. The software-as-a-service (SaaS) company supports regulated digital health innovation. Built under a strict QMS, the regulated IoT platform supports and maintains clinical-grade drugs, medical devices, combination products, and Software as a Medical Device (SaMD) development. Through smart connectivity, integrated data, and actionable insights, biopharma and medtech companies can optimize their therapies hosted on the BrightInsight Platform and drive improved patient adherence and engagement for enhanced outcomes at reduced costs.
With ongoing privacy, security, and regulatory compliance, the IoT platform provides the supporting medical-grade data infrastructure to enable its biopharma and medtech customers to accelerate digital innovation. BrightInsight adds more value to its innovator partners’ products through its modern and customizable platform architecture, pre-built software assets, and managed service that ensures ongoing compliance for its customer’s solutions hosted on the platform. Biopharma and medtech companies can develop, host, and manage connected, innovative digital health products and various data sources, e.g., combination devices, companion applications (app), SaMD, and smart algorithms, at scale.
Biopharma and medtech have to understand that digital is not a marketing concept, but the core of their value proposition. They can fundamentally unlock the right data and use it in a compliant manner to drive better outcomes, patient experience, cost savings, and ecosystem integration."
— Kal Patel, MD, CEO and Co-founder, BrightInsight
However, biopharma and medtech companies must first understand their vision and growth strategy. For instance, an innovator wants to incorporate Bluetooth into its insulin pen, a ‘simple drug delivery device,’ and a drug. They first need to build the data infrastructure that can support a regulated, connected combination product, and a platform that can analyze data to generate the desired insights, all while complying with the various privacy, security, and regulatory requirements.
Typically, developers do not realize they spend more time and money and increase risk by attempting to build the data infrastructure for their solution. This fundamental infrastructure is a more significant challenge than innovators expect when starting their journey.
BrightInsight: Leading Regulated IoT Platform
We [BrightInsight] are the plumbing. Highly regulated, highly compliant, highly secure plumbing that enables our biopharma and medtech customers to operate and build their innovation and solutions on top of it, including letting these partners work with each other through our common infrastructure."
— Kal Patel, MD
BrightInsight developed the leading global regulated IoT platform for biopharma and medtech by incorporating the skills and hard-learned lessons from their management team’s past experiences. The company built BrightInsight upon four critical pillars for successful digital health innovation.
Robust QMS: Only platform built on an ISO 13485:2016 certified QMS supporting the highest risk of intended use.
This certification demonstrates that BrightInsight operates a cutting-edge Quality Management System for software development services, such as developing and hosting SaMD and other regulated products. The company achieved certification per the ISO 13485:2016 standard, which aligns with the FDA’s quality system regulations principles and is recognized internationally, through independently audited processes, documentation, and quality management system.
Modular Architecture: Previously non-existent in biopharma and medtech, the unique regulated IoT platform allows for a common technology infrastructure that can integrate data across device types and manufacturers.
Unlike mostly archaic medical software, modern software relies on functional units related by use cases. Application programming interface (API)-led integration to its container-based microservices architecture design allows independent, self-contained service deployment, i.e., plug-and-play, agile, scalable, tailored, and seamless interoperability across apps, data sources, and devices.
Privacy and Security: Custodians of Protected Health Information (PHI), BrightInsight ensures privacy and security as demonstrated by various certifications, e.g., HITRUST, ISO/IEC 27001:2013, HIPAA and the EU’s General Data Protection Regulation’s (GDPR) compliance, EU-US and Swiss-US Privacy Shield frameworks, and the French Hébergeur de Données de Santé (HDS) certification.
The BrightInsight Platform supports GDPR requirements, such as a patient’s right to be forgotten. The system validates and implements the request at the backend, followed by completely deleting all the patient-related data according to GDPR requirements. The customer does not need to take action at any point in the execution.
If a government authority wants proof down the road of meeting privacy requirements, the platform’s detailed logging provides an audit trail to support the ask.
Fully-managed Service: End-to-end proactive infrastructure management enables BrightInsight’s customers to remain focused on their innovations.
The company bridges the gap for complex integrations and end-to-end infrastructure management. It helps innovators build their products on its infrastructure quickly. At the same time, BrightInsight performs ongoing maintenance and management, allowing innovators to remain compliant even after the product launch, providing assurance for its customers as privacy, security, and regulatory requirements evolve.
As part of its managed service, BrightInsight monitors and implements additional compliance requirements based on the country. Hence, it stays on top of all privacy and security requirements, while its customers continue to innovate and launch new products or trials around the world.
Customer-centric Solutions: A Winning Strategy
As the leading regulated IoT platform, innovators typically reach out to BrightInsight with an idea, such as building an algorithm that personalizes drug dosing or a connected combination product and supporting patient app. Customers license BrightInsight’s regulated IoT platform and, working side-by-side with its customers’ innovation development teams, the company’s software developers, regulatory experts, security experts, and project delivery team build the solution on top of BrightInsight’s infrastructure in a compliant manner.
What is unique about BrightInsight’s development process is its ability to develop software in an agile, fast-paced way while still maintaining compliance with regulatory, security, and privacy requirements and developing all of the supporting quality documentation. At the end of the development process, the company hands a final software product with the customer’s integrated code and intellectual property (IP) that leverages BrightInsight’s platform and various IPs, and a design history file (DHF). The design history file includes all the requirements, risk analysis, testing, traceability, and reference to the BrightInsight Platform. BrightInsight’s customers can leverage the DHF when they file their regulatory submissions for their digital health product, taking care of all of the supporting documentation for the software platform.
The company is beginning to bring partners to do custom professional services work on its customers’ behalf on top of its IoT platform, presenting more options for innovators.
Accelerated Innovation, Unprecedented Value
The BrightInsight Platform supports a broad set of devices and data sources of intended use cases. BrightInsight has projects across disease areas, including diabetes, obesity, respiratory, ophthalmology, neurology, and hematology, and modalities, such as devices, wearables, SaMD, algorithms, patient-facing apps, and physician-facing apps. All projects use the same underlying IoT platform infrastructure but are configured and customized for a specific application.
Akin to market shifts and trends, the company helps biopharma and medtech to execute cost-effective innovation—to enhance their therapies, accelerate clinical trials, and enable improved outcomes—saving time, money, and hassles. Looking at how real-world data can inform clinical endpoints will change how disease progression is determined and potentially enable more accurate, smaller, shorter, and cheaper clinical trials.
Today, we have customers working on everything from how to drive better patient engagement and adherence to therapy, to looking for potential adverse events for earlier intervention with higher specificity and sensitivity; both areas enable massively valuable interventions from a clinical outcome and cost savings perspective."
— Kal Patel, MD
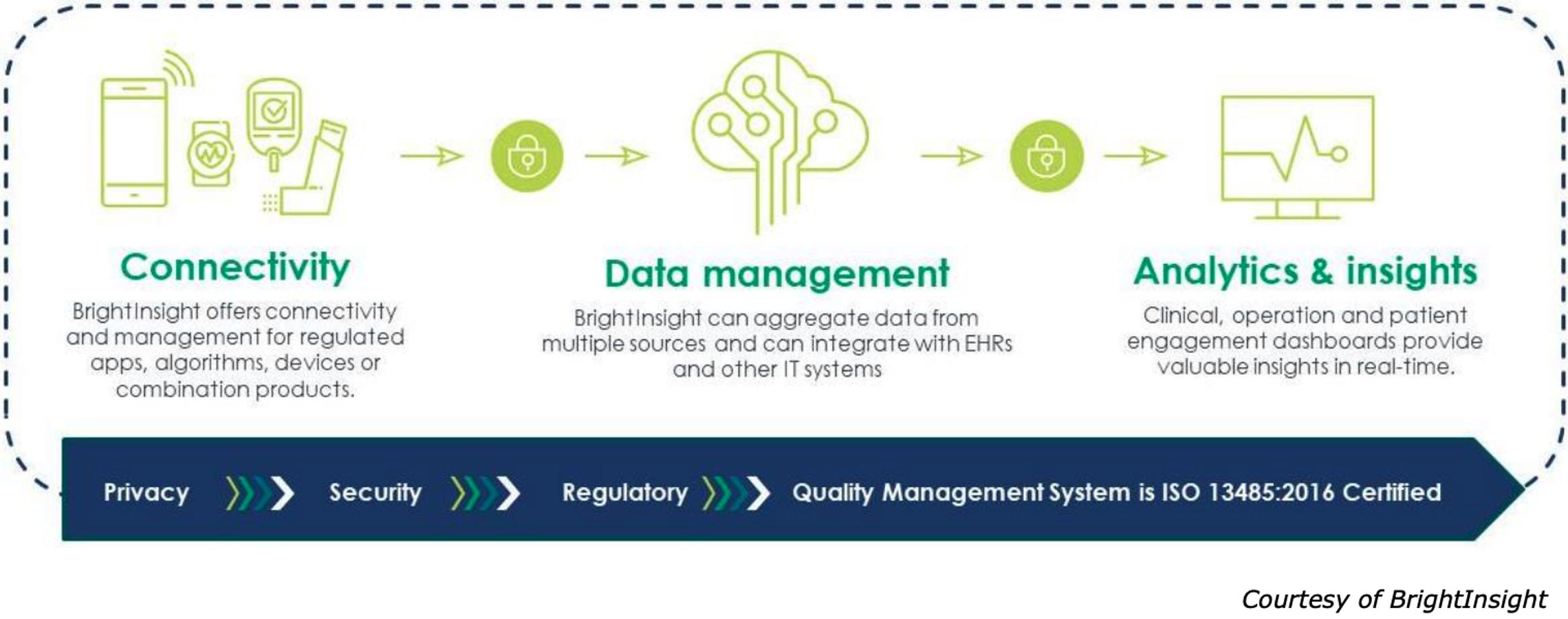
Applications and products from BrightInsight’s current customer base installs include:
Use Case 1: Class III Diabetes SaMD Algorithm
Problem: Currently, physicians make slow drug adjustments to a patient’s diabetes medication based on intermittent in-person interactions. With office visits every two to four months, it takes almost three years in the US and over two years in the EU to optimize patients’ long-term basal insulin dose. The technology and data exist to improve this process, but, mostly siloed, data integration and interoperability are lacking, crippling digital health innovation.
Solution: The patient titrates their insulin on their own based on data integrated from their continuous blood glucose meter, their connected insulin pen, and a Class III SaMD algorithm that tells a patient how to change their drug dose without a physician having to see the patient for intervention.
Value: A new patient gets up to its correct, personalized, long-term basal insulin dose in two to three months using a SaMD algorithm operating on the BrightInsight Platform.
Beyond speed to market, the BrightInsight Platform also adds significant value from its integrated ecosystem, e.g., data from a connected insulin pen only has value when holistic insights from continuous blood glucose meters are also available.
Integrating continuous blood glucose meter data, lifestyle and diet solutions, and so forth, provides comprehensive patient-generated health data and electronic patient reported (ePRO) outcomes. The regulated IoT platform’s common infrastructure extends data access and availability to all the stakeholders as needed/required by customers, i.e., provider, caregiver, and the patient itself, to optimize diabetes management and go-to-market strategies.
Fundamentally, solutions will always entail a combination of diagnostics, medical and behavioral interventions, and data-driven findings, all made possible by BrightInsight’s common data infrastructure."
— Kal Patel, MD
Use Case 2: Digital Health Suite of Products Focused on Patients with Rare Diseases
Problem: Many digital health innovations focus solely on the major disease categories affecting the largest patient populations. These innovations are important, but so are finding new ways to enhance care and treatments for patients with rare and serious diseases, especially in the time of COVID-19, when high-risk patient populations cannot engage the healthcare system as they typically would.
Solution and Value: A top biopharma company is developing digital health devices, apps, algorithms, and SaMD focused on improving the treatment experience for patients with rare diseases. They are building their innovative solutions on top of the BrightInsight Platform, aggregating data across their digital health solutions, and generating insights to enhance their offerings, all using the BrightInsight enterprise-wide IoT platform.
Use Case 3: Drug Dosing Calculation for Hemophilia A
Problem: Hemophilia A is a rare genetic disorder in which the bloodstream lacks sufficient specific clotting factors for blood to clot normally. Determining the correct drug dosage for an individual patient is often complicated.
Solution: BrightInsight launched a CE-marked Dosing Calculator to support the usage of a leading Hemophilia A drug. The novel digital tool enables healthcare professionals to input data to determine the correct dosage and number of vials needed for a patient based on the approved recommended dosage.
Value: The calculator aids physicians in determining the correct drug dose loading and maintenance to administer based on approved dosing recommendations and the patient’s biometrics inputs. Additionally, based on the same information, the Dosing Calculator also determines the recommended number of vial(s).
BrightInsight has ongoing discussions with biopharma and medtech companies around smart cardiac devices, enhancing data capture for patients enrolled in oncology clinical trials or using commercialized oncology therapies, immunomodulators, and more. Whether deployed on the edge, in a patient’s home, or at the clinic, innovators are also currently exploring patient triage use cases, harnessing patient generated data from implanted devices and ePRO automatically upon discharge for high fidelity longitudinal health assessments. Clinicians can track individuals requiring an earlier outpatient appointment than the one scheduled, improving outcomes while eliminating potential re-hospitalization.
BrightInsight bolsters bottom-up innovation across the industry. Like digital therapeutics, different disease areas and digital health solutions will coalesce around best practices and a common underlying infrastructure over time, advancing complex chronic disease management with personalized care.
Ahead of the Curve
BrightInsight’s regulated platform is gaining wide adoption across the industry. The company experienced double-digit revenue growth from 2018 to 2019. It expects to triple its business in 2020, with 100% growth rates in the years to follow.
The BrightInsight Platform’s privacy, security, quality, and regulatory compliance features are validated through key certifications, e.g., ISO 13485:2016 QMS, HITRUST, HIPAA, GDPR. With its sights set on standards globally, these certifications are central to its international expansion strategy to support its global customers. In the next 12 to 24 months, BrightInsight will transition from creating a new category to leading the segment, to becoming the gold standard in the market.
Frost & Sullivan believes that BrightInsight provides a sustainable competitive advantage, strengthening the SaaS model and its status as the leading global regulated IoT solution. BrightInsight is well-poised to capture significant market share in the fast growing emerging segment.
Digital technologies play a crucial role in value-driven care and are becoming commonplace across applications and disease areas. Nonetheless, data integration and interoperability are lacking, limiting digital health innovation. Biopharma and medtech companies demand widespread ecosystem integration and require privacy, security, quality, and regulatory compliance, all amidst ongoing software changes inherent to digital health products. BrightInsight ensures compliance at each step to accelerate cost-effective innovation for its biopharma and medtech clients.
BrightInsight’s leading global regulated Internet of Things (IoT) platform, the BrightInsight Platform accelerates regulated digital health innovation for biopharma and medtech through a common language and technology infrastructure. Flexible, fast, and secure, the IoT platform supports high value-added, and highly regulated, digital innovation. End-to-end enablement, monitoring, and management are driving BrightInsight’s increased adoption. BrightInsight accelerates innovation speed dramatically and reduces both the risk and cost substantially.
With its strong overall performance, BrightInsight earns Frost & Sullivan’s 2020 Global Entrepreneurial Company of the Year Award for healthcare IoT solutions.
Ultimately, growth in any organization depends on customers purchasing from a company and then making the decision to return time and again. In a sense, then, everything is truly about the customer. Making customers happy is the cornerstone of any successful long-term innovation or growth strategy. To achieve the dual goals of customer engagement and growth, an organization must be best in class in 3 key areas: understanding demand, nurturing the brand, and differentiating from competition.
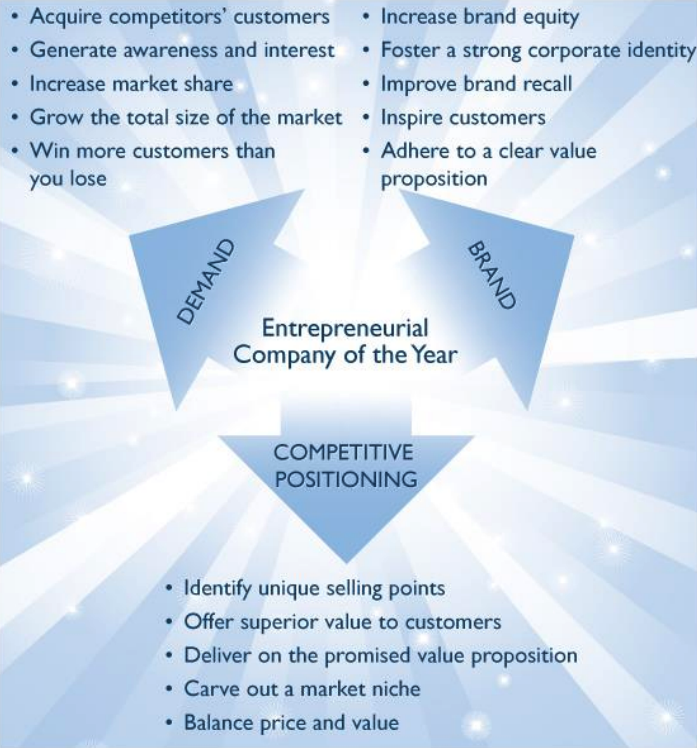

Demand forecasting, branding, and differentiation underpin an entrepreneurial company’s journey toward forming deep relationships with customers and permanently altering the market with their actions. Entrepreneurial Innovation and Customer Impact are the cornerstones of this award, as discussed further in the next section.
For the Entrepreneurial Company of the Year Award, Frost & Sullivan analysts independently evaluated Entrepreneurial Innovation and Customer Impact according to the criteria identified below.
Criterion 1: Market Disruption
Requirement: Innovative solutions that have genuine potential to disrupt the market, obsoleting current solutions and shaking up competition
Criterion 2: Competitive Differentiation
Requirement: Deep understanding of both current and emerging competition to create and communicate strong competitive differentiators in the market
Criterion 3: Market Gaps
Requirement: A clear understanding of customers’ desired outcomes, the products that currently help them achieve those outcomes, and where key gaps may exist
Criterion 4: Blue Ocean Strategy
Requirement: Strategic focus on creating a leadership position in a potentially "uncontested" market space, manifested by stiff barriers to entry for competitors
Criterion 5: Passionate Persistence
Requirement: A deep belief in the “rightness” of an idea and a commitment to pursuing it despite seemingly insurmountable obstacles
Criterion 1: Price/Performance Value
Requirement: Products or services offer the best value for the price, compared to similar offerings in the market.
Criterion 2: Customer Purchase Experience
Requirement: Customers feel they are buying the most optimal solution that addresses both their unique needs and their unique constraints.
Criterion 3: Customer Ownership Experience
Requirement: Customers are proud to own the company’s product or service and have a positive experience throughout the life of the product or service.
Criterion 4: Customer Service Experience
Requirement: Customer service is accessible, fast, stress-free, and of high quality.
Criterion 5: Brand Equity
Requirement: Customers have a positive view of the brand and exhibit high brand loyalty.
Frost & Sullivan analysts follow a 10-step process to evaluate Award candidates and assess their fit with select best practice criteria. The reputation and integrity of the Awards are based on close adherence to this process.
Frost & Sullivan’s 360-degree research methodology represents the analytical rigor of our research process. It offers a 360-degree-view of industry challenges, trends, and issues by integrating all 7 of Frost & Sullivan's research methodologies. Too often companies make important growth decisions based on a narrow understanding of their environment, leading to errors of both omission and commission. Successful growth strategies are founded on a thorough understanding of market, technical, economic, financial, customer, best practices, and demographic analyses. The integration of these research disciplines into the 360-degree research methodology provides an evaluation platform for benchmarking industry participants and for identifying those performing at best-in-class levels.


Frost & Sullivan, the Growth Partnership Company, enables clients to accelerate growth and achieve best-in-class positions in growth, innovation and leadership. The company's Growth Partnership Service provides the CEO and the CEO's Growth Team with disciplined research and best practice models to drive the generation, evaluation and implementation of powerful growth strategies. Frost & Sullivan leverages more than 50 years of experience in partnering with Global 1000 companies, emerging businesses, and the investment community from 45 offices on six continents. To join our Growth Partnership, please visit http://www.frost.com.