
This international standard, published in 2016, applies to the safety and security of health software products designed to operate on general computing platforms and intended to be placed on the market without dedicated hardware, and its primary focus is on the requirements for manufacturers.
Where BrightInsight is the Legal Manufacturer of an SaMD, this standard is applied in the development of the SaMD.
The figure below (from the standard) indicates the scope of the standards IEC 82304-1 and IEC 62304.
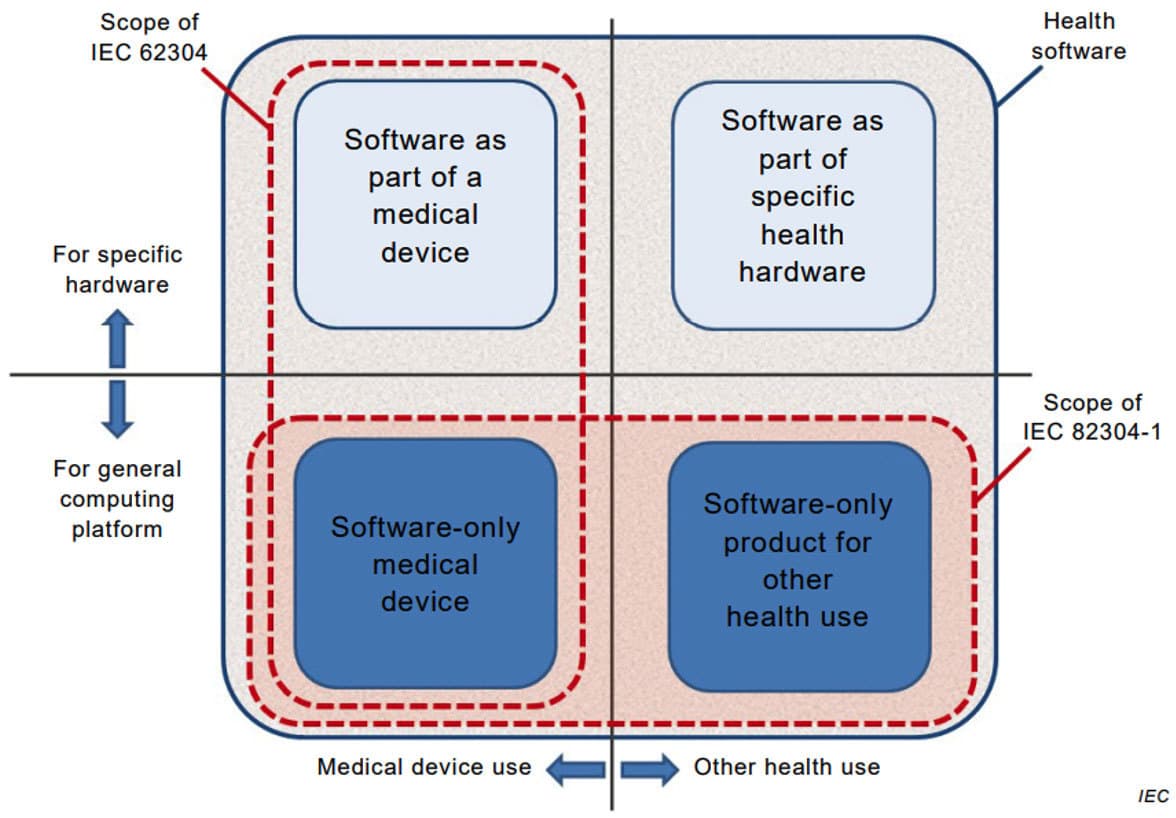
IEC 82304-1 requires and builds on IEC 62304. The figure below, also from the standard, indicates the Health Software product processes.
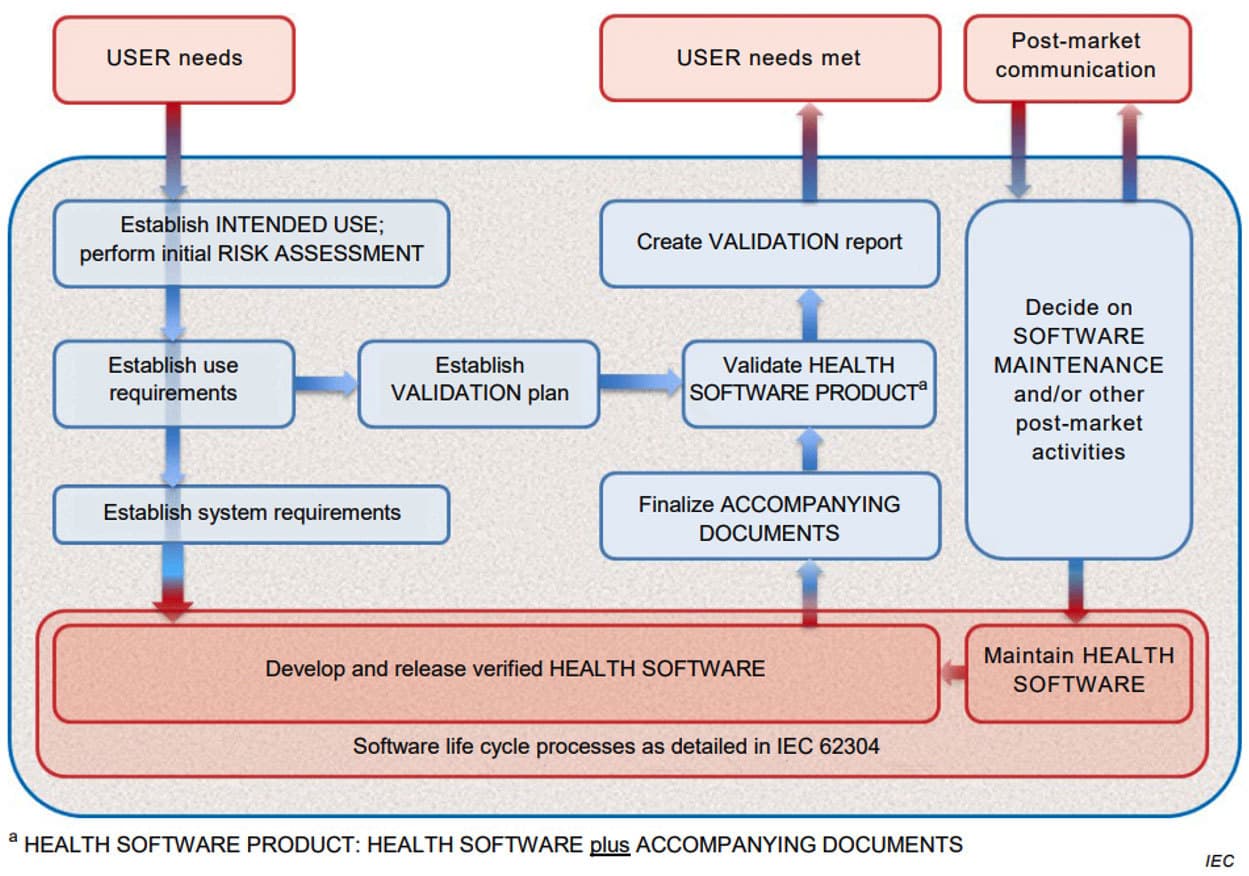
The clauses of IEC 82304-1 aim to define a framework for the design of the standalone software system, the user documentation and labeling, and for post-market activities.
Although IEC 82304-1 is not yet required or expected by regulators, application of the standard has been adopted by BrightInsight to align with industry best-practices.