
Case Study
Hemophilia is a rare genetic condition in which the liver doesn’t produce sufficient amounts of a protein that supports blood clotting. The number of people living with hemophilia worldwide is up for debate, as the condition often goes undiagnosed. A 2019 study published in the Annals of Internal Medicine estimated that roughly 1,125,000 people around the world—almost entirely men—have this inherited bleeding disorder.1
Rarer still, only about 15% of all patients with hemophilia have hemophilia B—defined by a lack of Factor IX clotting factor, specifically. Patients with this condition live in constant fear that even a minor injury or medical procedure could turn into a life-threatening bleeding event. Over time, limited mobility, joint damage and severe pain affect quality of life. Up until recently, the standard of care was lifelong prophylaxis treatment, consisting of routine infusions of Factor IX clotting factor.
CSL Behring’s groundbreaking gene therapy, HEMGENIX®, was approved by the FDA in November of 2022 and has the potential to revolutionize how patients and their providers manage this burdensome condition.
As the result of 20 years of research, HEMGENIX is the first and only FDA-approved gene therapy for hemophilia B. Not only does it provide significant bleed protection versus Factor IX prophylaxis, but because the treatment is given via a one-time infusion, it also has incredible promise for improving quality of life.

Despite the promise of this game-changing treatment, hurdles on the patient journey to gene therapy are significant. Some patients may be hesitant to switch from a treatment plan they’re comfortable with. Others may be uncomfortable with gene therapy overall.
These were just some of the challenges CSL Behring was facing when they called upon BrightInsight. With CSL Behring's 30-plus years serving the hemophilia community and extensive market knowledge, along with intensive market research and a deep bench of industry experts, the companies studied the unique needs of hemophilia B patients and worked in partnership to roll out a comprehensive mobile companion app for not only patients using HEMGENIX, but for all patients treated with Factor IX replacement therapy.
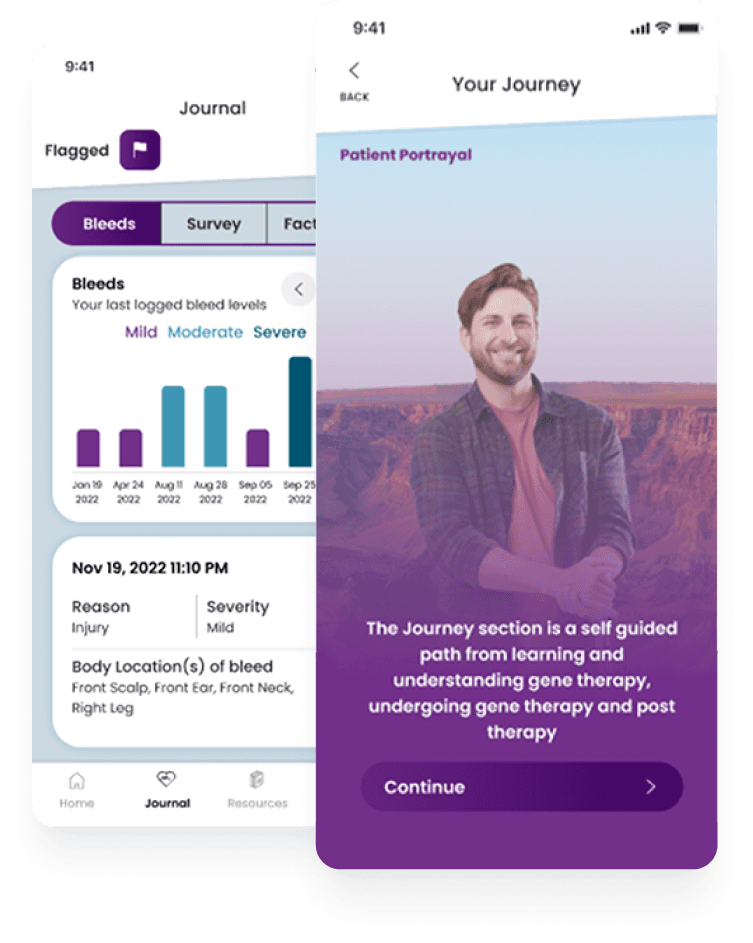
Released in April 2023, B SUPPORT is a comprehensive mobile app that meets patients wherever they are in their hemophilia B treatment journey with a suite of features, from tailored educational materials to incident tracking and enhanced communication with CSL Behring's support team.
Like many other rare and serious diseases, managing hemophilia B can be incredibly taxing on patients. Long-term prophylaxis treatment can be hard on the body. Patients on regular prophylaxis are at risk for vein collapse, the result of up to 156 infusions each calendar year. What’s more, even those on prophylactic treatment may still experience spontaneous bleeds and joint bleeding.
The psychological burden shouldn’t be overlooked, either. A study in the journal Patient Preference and Adherence showed a relationship between depression, anxiety, pain and treatment adherence in hemophilia.2,3
While the primary audience for the B SUPPORT app is people considering switching to HEMGENIX (and those early adopters who have already completed treatment), it was important to CSL Behring that the app support patients in all stages of the hemophilia B journey—including those who may be far from ready to consider a gene therapy. Therefore, the B SUPPORT app was created to help solve significant patient challenges across the care continuum, including:
2 CSL Behring. New Survey Spotlights Ongoing Concerns for People Living with Hemophilia B. HEMAWARE: The Bleeding Disorders Magazine. Accessed February, 2023.
3 Witkop ML, Lambing A, Nichols CD, Munn JE, Anderson TA, Tortella BJ. Interrelationship between depression, anxiety, pain, and treatment adherence in hemophilia: results from a US cross-sectional survey. Patient Preference and Adherence. November 2022.
4 Alshaikhli A, Rokkam VR. StatPearls: Hemophilia B. National Center for Biotechnology Information. Last updated August 2023.
CSL Behring engaged BrightInsight to create a companion app for people living with hemophilia B that could launch right on the heels of the HEMGENIX FDA approval, meeting three key objectives:
In order to accelerate speed to market and launch quickly after FDA approval, BrightInsight took a progressive release approach, first determining the minimum viable product: a patient-friendly, highly usable app that includes core features for patients considering a move to HEMGENIX.
This includes features like:
And since no two patient journeys are the same with hemophilia B, the app features a dynamic home screen. Based on where they are in their treatment journey, recent bleed events and other factors, the app customizes what each user sees on their home screen, ensuring that the app continues to be a helpful tool in their disease management progress.
Future releases of the app will include additional community-focused features, support for more languages and other improvements based on the invaluable data collected. It will also include content and features for users earlier in the consideration phase who may not yet be aware of gene therapy as a better treatment option.
We worked closely with BrightInsight to develop an app that met the diverse needs of hemophilia B patients. With CSL Behring’s deep market knowledge and long history serving this community and BrightInsight’s astute understanding of patient needs and behaviors, we carefully studied the patient journey to identify touch points we could impact with digital. Together we were able to build tools that guided patients through this unique journey in a very holistic way."
David Chu, Director of Marketing, HEMGENIX

The BrightInsight Platform offers highly configurable, flexible functionality that allows us to launch apps that meet the specific needs of life sciences companies in as few as six months. Key features include:
After the success of our first companion app for Hizentra®, which earned high adoption and retention, CSL Behring signed an enterprise licensing agreement with BrightInsight to scale digital initiatives across their brand portfolio. HEMGENIX is the second CSL Behring therapy to launch an app with BrightInsight within this partnership. Our platform is deployment-ready in 64 countries, which allows companies like CSL Behring to scale globally. The company expanded the Hizentra app to Japan and plans to continue the global expansion with planned launches in Canada, South America and Central America.
We partnered with BrightInsight to develop standardized app modules that we can pull off the shelf and put a new brand coat of paint on it. And it saves us a ton of time and money in development. The app modules include features like an ePRO, calendar support, patient login — all components that we need for every single app. Instead of starting from scratch for each app, we can leverage BrightInsight’s pre-built app modules. Then our development time is focused just on the new and unique features. This approach is more efficient for us from a cost and time perspective."
Brian Johnson, Senior Director, Customer Engagement Management at CSL Behring
CSL Behring is a global biotherapeutics leader driven by its promise to save lives. Focused on serving patients’ needs by using the latest technologies, the company discovers, develops and delivers innovative therapies for people living with conditions in the immunology, hematology, cardiovascular and metabolic, respiratory and transplant therapeutic areas. CSL Behring uses three strategic scientific platforms of plasma fractionation, recombinant protein technology, and cell and gene therapy to support continued innovation and continually refine ways in which products can address unmet medical needs and help patients lead full lives.
CSL Behring operates one of the world’s largest plasma collection networks, CSL Plasma. Its parent company, CSL Limited (ASX:CSL; USOTC:CSLLY), headquartered in Melbourne, Australia, employs more than 25,000 people, and delivers its life-saving therapies to people in more than 100 countries. For inspiring stories about the promise of biotechnology, visit CSLBehring.com/Vita and follow us on Twitter.com/CSLBehring.
BrightInsight provides the leading global platform for biopharma and medtech regulated digital health solutions. When speed matters, we help companies accelerate time to market for regulated digital health offerings across therapeutic areas, including apps, healthcare provider interfaces, analytics dashboards, algorithms, medical devices, connected combination products, diagnostics and Software as a Medical Device (SaMD).
BrightInsight replaces the need for lengthy and complex “build from scratch” implementations by offering configurable applications and a proven platform built under a Quality Management System to support global security, privacy and regulatory requirements. When building digital health products on the BrightInsight Platform, compliance is future-proofed as intended use changes scale across geographies.