Flex Digital Health, Inc. has been awarded the International Organization for Standardization (ISO) 13485:2016 certification for medical device quality management systems.
Flex Digital Health’s core product offering, BrightInsight, is a medical-grade, intelligent platform that optimizes connected drug, device and combination products through real-time, integrated data and actionable insights to enable customers to drive increased patient adherence and engagement. Deployed as a managed service, BrightInsight is designed to support CE-marked and FDA-regulated Class I, II and III medical device, combination product and Software as a Medical Device requirement.
This ISO certification is another milestone for Flex Digital Health following the acceptance of its master file by the FDA for the BrightInsight Platform in March of 2018.
“Flex Digital Health is committed to providing medical-grade solutions for our pharma and medtech customers who operate in highly regulated industries, and our ISO 13485:2016 certification is another affirmation of this commitment.” said Michael Righter, Senior Director, Regulatory Affairs at Flex Digital Health.
Digital-savvy pharma and medtech companies are adding new regulated digital health capabilities – such as controlling connected devices, drug dosing, decision support and automated interventions -- as a way to improve patient engagement, deliver actionable insights to providers, and provide more value around their products and therapies.
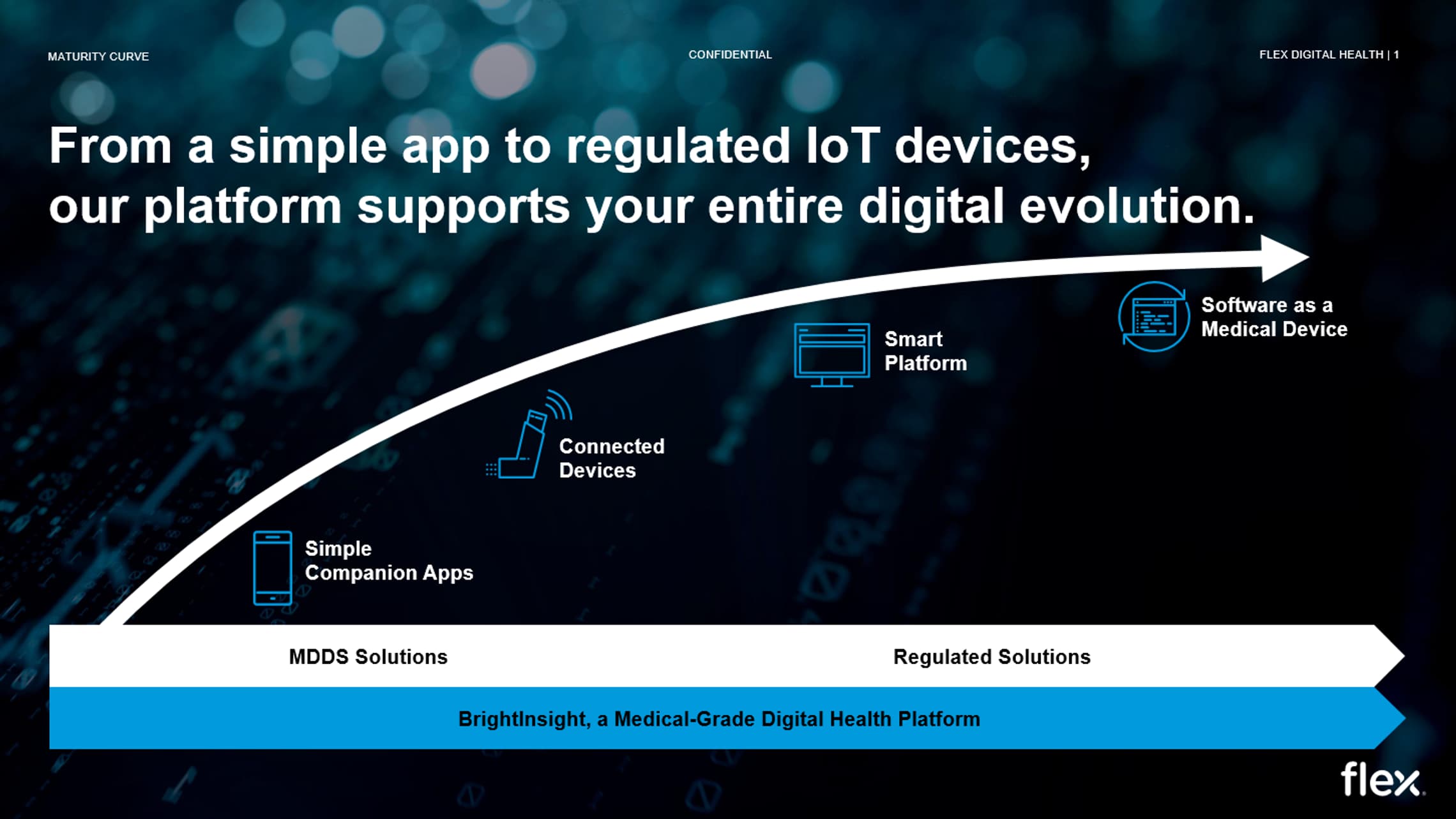
As companies transition from developing simple companion apps, to capturing data from connected medical devices, to building Software as a Medical Device solutions, they need a compliant infrastructure and regulatory expertise to ensure their products are designed and maintained in an environment that is compliant with international quality and regulatory standards. Building these capabilities within a pharma or medtech company impacts their time to market, increases headcount costs, and introduces additional regulatory and audit exposure.
Our BrightInsight Platform can support our customers’ advanced use cases across the digital health regulatory maturity curve.
BrightInsight is built from the ground up to securely manage highly regulated medical device data and personal health information. Flex Digital Health has put the people, technology and processes in place to monitor security and threat prevention to meet global compliance standards.
Read the original post on flex.com.