

64
countries are deployment ready
~$35B
of combined forecast for peak annual sales for drugs and IVD assets on our platform
80%+
disease states covered

CSL Behring leveraged the BrightInsight Platform® to launch a mobile app for adult patients with rare diseases who are taking Hizentra.
Read Now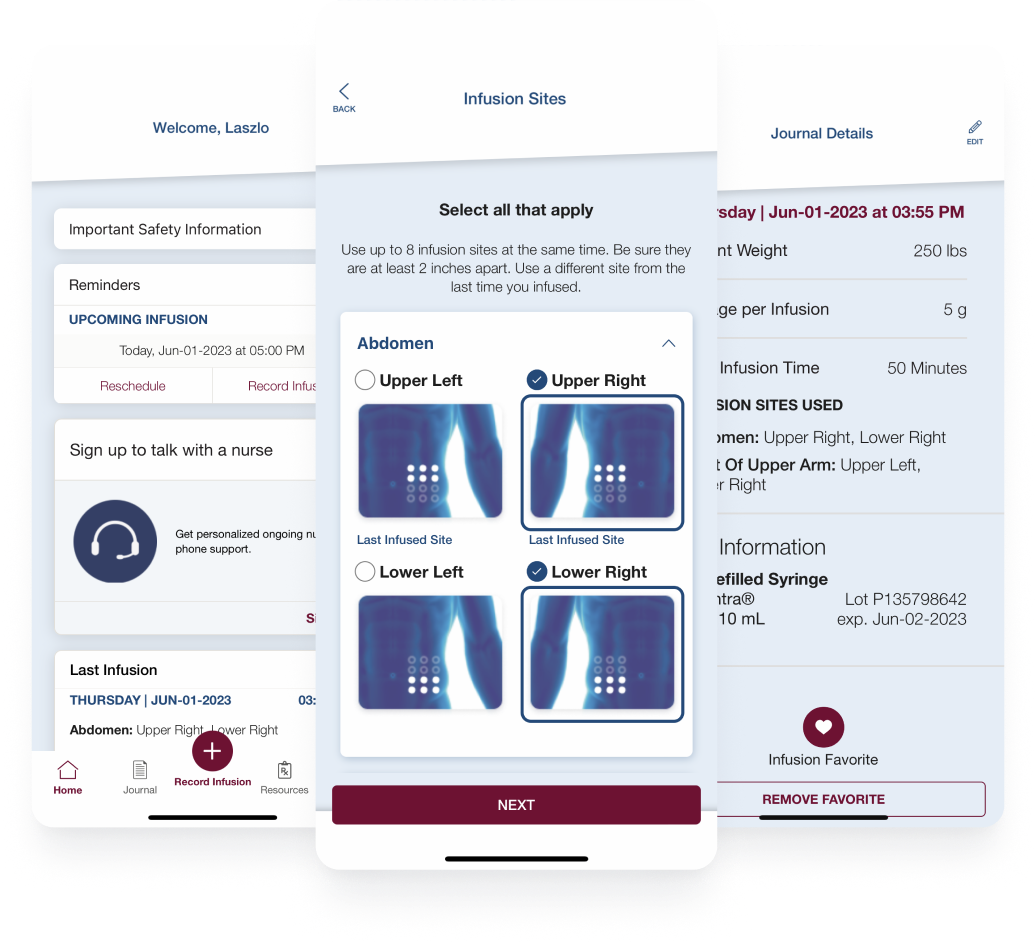

Contact us today to start a conversation about how BrightInsight can help on your digital health journey.
Contact us